Anyone who has been through a major surgery knows the importance of pain management. We are all aware of the benefits and downsides of opioid pain medication. Although extremely effective, opioids can also be highly addictive. Thanks to some innovative biotech companies, there are new alternatives that provide the benefits without the downsides.
HHS.gov/opioids provided the following infographic to illustrate the problem.
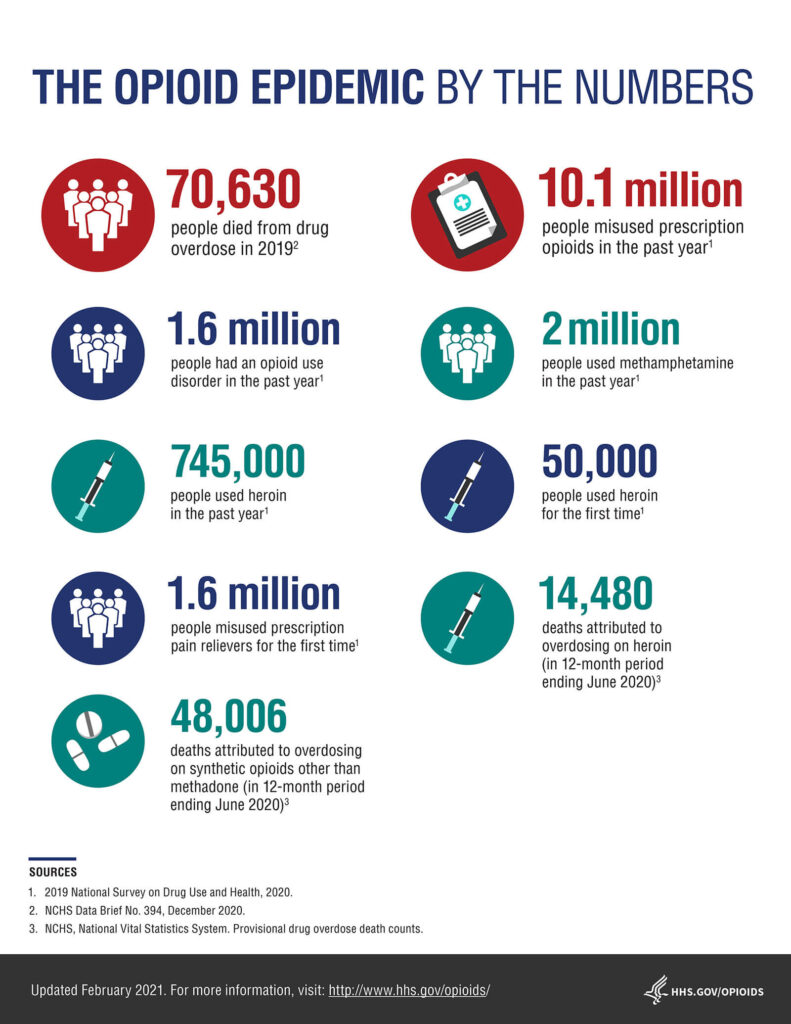
Shining a spotlight on some innovative biotechs developing non-opioid alternatives to pain relief.
Pacira, San Diego, CA, The cornerstone of the Pacira product portfolio is DepoFoam®, a proprietary drug delivery platform designed to extend a medication’s duration of action without altering its molecular structure. The DepoFoam carrier matrix is made up of multivesicular liposomes that encapsulate a drug. Each chamber is separated by lipid membranes that naturally erode to release the drug over a desired period of time.
In 2012, Pacira successfully launched EXPAREL® (bupivacaine liposome injectable suspension), the first and only DepoFoam-based local analgesic. Indicated for administration into the surgical site to produce postsurgical analgesia, a single dose of EXPAREL provides non-opioid pain control with reduced opioid requirements for up to 72 hours* without the need for catheters or pumps.
WEX Pharmaceuticals Inc. Vancouver, Canada, is developing a new class of non-opioid analgesics. WEX’s proprietary platform and the lead product is Halneuron®. The active pharmaceutical ingredient in Halneuron is Tetrodotoxin (“TTX”) which is a proven sodium channel blocker.
Halneuron is in Phase III clinical development for the treatment of cancer-related pain and will commence Phase III clinical development for chemotherapy-induced neuropathic pain. WEX has conducted over 15 clinical trials with TTX and has treated more than 700 patients to date.
Regulonix, Arizona, has designed a drug that disrupts the interaction between a mediator protein called CRMP2 and an enzyme, Ubc9, which is important in the activity of NaV1.7. The drug reversed pain in six different rat models, the researchers showed in a study published in Science Translational Medicine.
The University of Arizona has licensed the drug which was co-founded by the study’s senior author, Rajesh Khanna, Ph.D., to develop ion channel-targeting non-opioid painkillers. Khanna, a professor of pharmacology, and his team have been working with the National Institutes of Health’s National Center for Advancing Translational Sciences to optimize the compound, according to the university’s health sciences department.
Concentric Analgesics, San Francisco, CA is a clinical stage biotechnology company committed to developing novel, non-opioid therapeutics for chronic pain management. The company’s lead drug candidate CA-008 is a water-soluble prodrug that is a potent TRPV-1 agonist and can selectively and reversibly desensitize pain conducting C-fiber nociceptors. It has the potential to eliminate the need for opioids in post-surgery recovery period and can offer pain relief up to a week after injecting.
The company is focused on three underserved markets namely management of post-surgical pain, chronic osteoarthritis pain, and intractable cancer pain. The company received a Breakthrough Therapy designation from the U.S. Food and Drug Administration (FDA) for CA-008 in post-surgical pain in September 2018.
